About Us
At epm Therapeutics we’re driven by a singular goal: to improve the lives of Prader-Willi syndrome (PWS) patients and their caregivers through groundbreaking science. For those living with PWS, the routine of their lives and their caregivers’ lives are often interrupted by a relentless cycle of hunger, anxiety, and exhaustion.
But what if there was a way to disrupt this cycle?
Imagine more PWS patients feeling satiated after a meal.
Imagine caregivers focusing on improving quality of life and well-being, not reprimanding or isolating the patient
Imagine a treatment that targets hyperphagia and the crippling anxiety and fatigue that compound the suffering.
Our Story:
Science with a Human Purpose
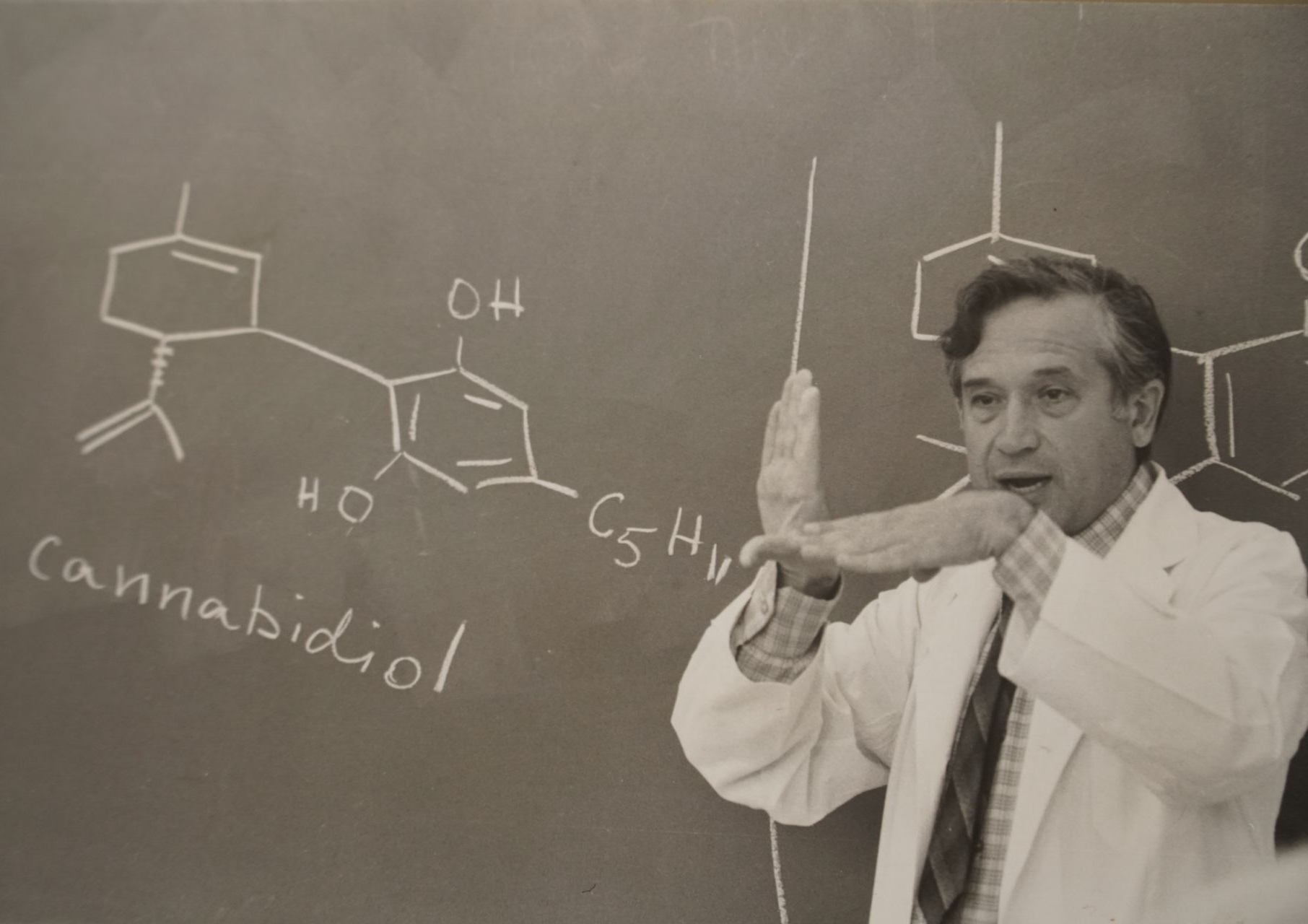
EPM Group Inc. dba epm Therapeutics was founded in 2017 by partnering with Hebrew University of Jerusalem and the late Professor Raphael Mechoulam (a luminary in cannabinoid research). Dr. Mechoulam had successfully isolated, identified, and stabilized Cannabidiol Acids (CBDA). CBDA has a distinct pharmacokinetic and receptor affinity profile when compared to CBD. epm licensed and patented a portfolio of 14 different CBDA molecules for development.
Based on Dr. Mechoulam's early work and our own development program, we quickly focused on EPM301 and proceeded to pursue indications in Prader-Willi syndrome (PWS). We are proud to honor Dr. Mechoulam's science, vision, and legacy by developing EPM301 as a potential treatment for multiple symptoms of PWS.
Dr. Raphael Mechoulam
"Father of Cannabis Research", 1930 -2023
EPM301 can be that treatment. By addressing the common symptoms of PWS, we’re offering science-inspired hope for patients and caregivers alike based on positive in-vitro and animal model study results. Our lead candidate, EPM301, shows activity to improve multiple debilitating symptoms of PWS—hyperphagia (uncontrolled appetite), daytime sleepiness, and anxiety—offering hope where few treatments exist. epm Therapeutics' mission isn’t just about developing a medicine; it’s about improving independence, safety, and quality of life for patients—and relieving the extraordinary burden on the families and caregivers who devote their lives to those affected with PWS.
Why PWS? Why Now?
EPM301 has been granted an Orphan Drug Designation (ODD) status and Rare Pediatric Disease (RPD) designation with the possibility of a Priority Review Voucher upon approval by the US Food & Drug Administration (FDA) as well as ODD status with the EU’s European Medicines Agency (EMA)
- Addressing multiple and potentially life-threatening symptoms with high caregiver burden
- $500M+ US market potential with limited available treatments.
There are approximately 18,000 PWS patients living in the U.S., and the market potential is forecasted to exceed $500M. There are approximately the same number of patients in the E.U.
Invest With Impact
For investors, epm Therapeutics represents an opportunity to back our novel science toward a profoundly positive human impact. PWS has no cure, but with EPM301, we’re researching some of its most devastating symptoms. The goal is to redefine what’s possible for PWS patients, their families and their caregivers.
